Viscosity – more than just “liquid”
Viscosity is the measure of the toughness of liquids and gases. This can be understood as the work that is required to deform liquids or to allow them to flow at a certain speed.

Figure 1 The term viscosity is derived from bird glue, which was previously made from mistletoe. The plant genus mistletoe has the scientific name “Viscum”.
Isaac Newton recognised the proportionality between flow speed and deformation force in liquids. He summarises this in Newton’s Law:
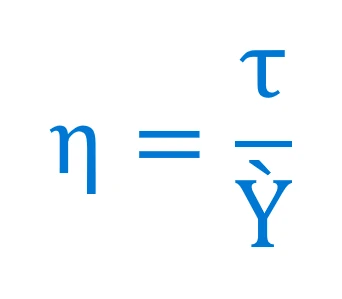
Fluids that adhere to this law, e.g. water or air, are called Newtonian fluids.
Behaviour that deviates from this is called non-Netonian and is the subject of rheology.
Examples of non-Newtonian liquids are polymers, hot-melt adhesives, but also blood or ketchup. The deformation behaviour of such liquids can no longer be simply described using Newton’s law (see above).
The viscosity of non-Newtonian fluids changes in particular with the shear rate or the shear stress, taking into account the duration of load (time of force application).
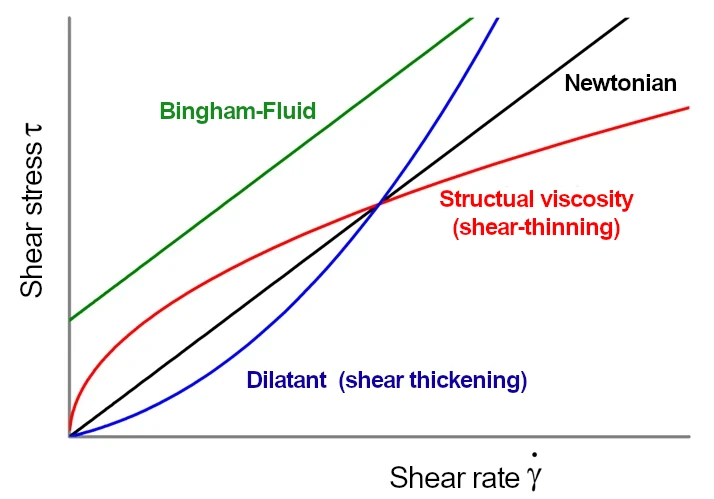
Hot-melt adhesives are non-Newtonian liquids because they are polymers or have a high polymer content. These polymers orient themselves in the direction of flow. The alignment in the flow direction itself requires time (load duration) and the orientation reduces the necessary force (shear stress).
The viscosity of a hot-melt adhesive is an essential selection criterion for successful application. The flow behaviour must, for example, be taken into account in running speeds and the timing of the application. The same applies to vertical or horizontal application or spray applications.
The viscosity is also correlated to the cohesion of a hot-melt adhesive, in general it can be said that the higher the viscosity, the greater the cohesion of the adhesive.
Here are some examples of viscosity values:
|
Water (20 °C) |
1 mPas |
|
Blood |
4 bis 25 mPas |
|
Engine oil |
~100 mPas |
|
Honey |
~10.000 mPas |
|
Glass |
~10.000.000.000 mPas |
