Adhesion
Adhesion describes the ability of two substances to stick together. It is the sum of all the attractive forces that act between two different material surfaces.
These are mainly physical interactions (Van der Waals forces, hydrogen bridges, etc.).
In the case of reactive hot-melt adhesives, chemical bonds to the substrate surface can occur. In comparison, these are much stronger than the physical interactions of non-reactive hot-melt adhesives.
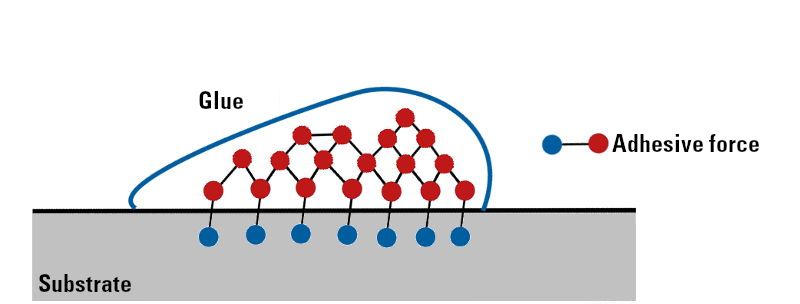
Figure 1: Explanation of adhesion
For the formation of adhesion, the physical interaction between adhesive and substrate is the essential prerequisite; the adhesive and the adherend/part to be joined must come closer than 1 nm within the open time of the adhesive.

Figure 2: Physical interaction between adhesive and substrate
The particular challenge in the case of hot-melt adhesives is the rapidly increasing viscosity after application. This then makes wetting und thus bridging of the aforementioned 1 mm to the second part to be joined more difficult.
In addition to joining within the open time, pressure on the substrates to be joined is an important factor when bonding with hot-melt adhesives.
Mechanical “adhesion” or mechanical anchoring can make an additional contribution. Here it is assumed that the adhesive penetrates into tiny pores and unevenness of the adherend and thus adheres to it.
Adhesion is still being scientifically researched and is currently described using different theories, as the dependencies between the adhesive systems and the surfaces of the parts being joined are extremely complex.
